Quality - Certifications
ISO 9001 - ISO 13485 - CEAn overall desire for continuous improvement
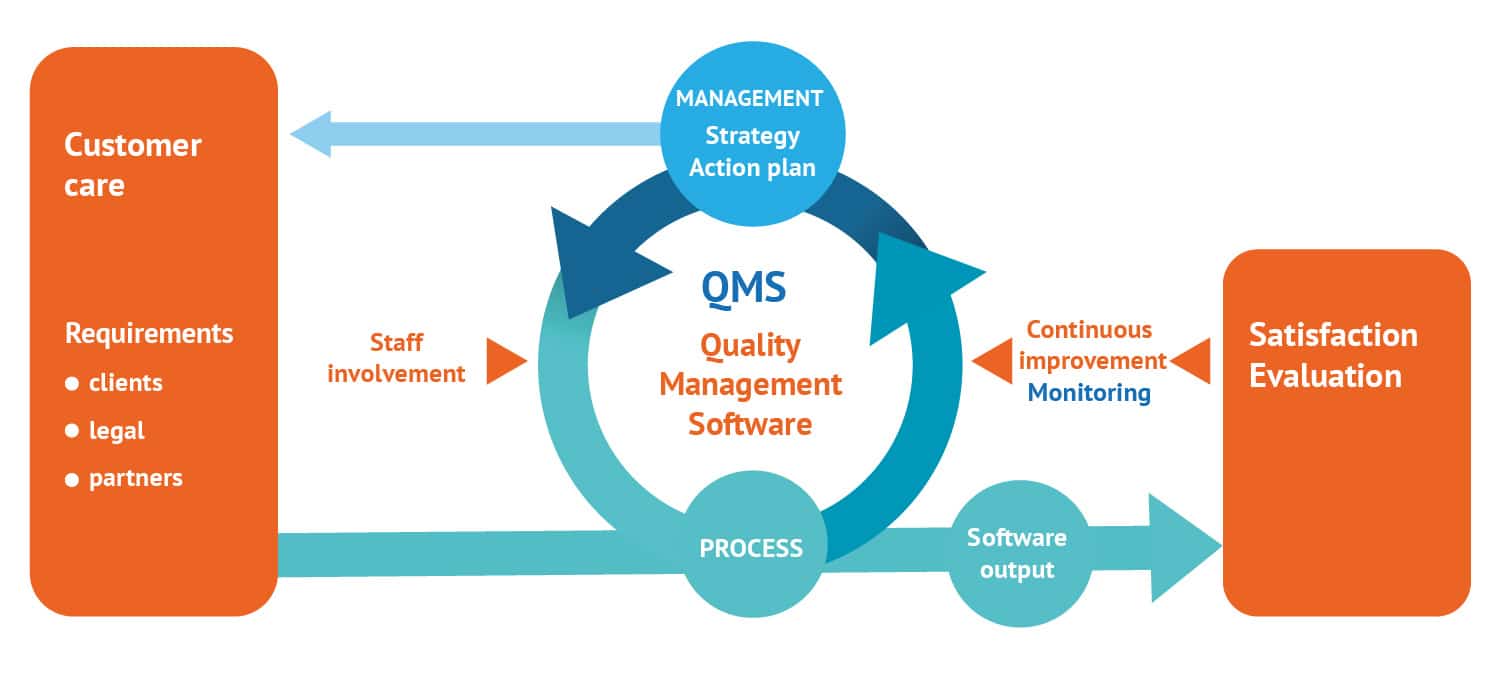
In 2014, Evolucare implemented a Quality Management System compliant with ISO 9001 and ISO 13485 standards. The purpose of this initiative is to define an internal organization for the company in order to streamline operations, continuously improve client satisfaction and meet regulatory requirements such as CE marking. These different reference systems are constantly changing.
Consequently, this continuous improvement approach is now being supplemented by action plans aimed at compliance with the new European regulation for medical devices.
“The Quality team works with all the department managers on all the group’s processes, from sales to customer service, including purchasing and production.”
Morgane le Guilcher,
Director of Quality – Safety – Environment
New European regulation
The new European regulation, called MDR 745/2017, is a major change.
Indeed, CE marking was based on the European directive 93/42/EEC, applicable since 1993 and transposed into the legislation of each European country. This directive has now been replaced by a mandatory European regulation effective as of May 26, 2021. It is stricter and more comprehensive than the directive.
Our Quality team is working to adapt our QMS accordingly, to prepare the marking of our products based on this new regulation and to guarantee our clients the safety of our products by reinforcing the post-marketing supervision of software dedicated to the monitoring of clinical performance, as required by the MDR.
“Our risk management method has been enhanced as a result of the new regulation, which places special emphasis on benefit/risk analysis.”
Sébastien Decaix, Quality Production Project Manager
Cybersecurity
Cybersecurity is now a key issue in the healthcare sector. Evolucare is committed to protecting sensitive medical data with solutions that comply with the most stringent standards. Find out how we integrate security into the design of our software.
Our quality policy
- The continuous improvement of the Group’s operational performance
- The continuous improvement of our customers’ satisfaction
- The consolidation of communication and cohesion among the Group’s teams
- The desire to expand internationally
- The development of skills through the continuous training of our employees
- The continuous evolution of the methodologies and tools used
- The reinforcement of rigour in the deployment and maintenance of our solutions
- The consideration for quality of life at work and safety
- The understanding of and compliance with our processes by our employees, sub-contractors and partners
- The development of innovative products
Labels & certifications
Evolucare Technologies is ISO 9001: 2015 and ISO 13485: 2016 certified, and meets the regulatory requirements for CE marking of medical devices:
- Osiris, our Patient Record, is a Class I medical device,
- Our critical care software, Evolucare Anesthesia and Evolucare Intensive, are Class IIa,
- Evolucare Imaging, our RIS-PACS solution, is Class I,
- Evolucare Uview/OphtAI is a Class IIa medical device.
Evolucare is certified ISO / IEC 27001:2017 compliant – Health Data Hosting (HDS) Outsourcer – LSTI Certificate N°11278-2 for the products and services concerned. The scope of the ISMS covers the development, provision and outsourcing of hosted applications.


All the news of the Quality activity

Evolucare OphtAI CE certified according to the MDR
The safety and reliability of medical devices are major challenges in the digital transformation of the healthcare sector.

ISO 14971: manage risks, drive innovation
Software security is not a constraint, it is a collective strength that promotes trustworthy digital health. In the digital health sector, the security of software solutions is essential. At Evolucare, this requirement is integrated from the earliest stages of ...

MFA, DMP, Ségur VA2: things are heating up!
Healthcare establishments can no longer put off the digital shift Summer isn't even over yet, and the new school year is already shaping up to be a tense one for educational institutions. The focus is on the widespread adoption of strong authentication in ...

Quality service at Evolucare: a strategic pillar for excellence
Ensuring quality: a major challenge in digital health In an industry where safety, reliability, and compliance are essential, quality is not just a regulatory requirement: it is a prerequisite for ensuring safe and effective solutions.At Evolucare, our quality ...

Medical devices: Evolucare obtains MDR certification
INTENSIVE+ A major step forward for digital healthcare: Evolucare is now MDR-certified for all its Medical Devices. We are proud to announce that our portfolio of medical device products has received MDR (Medical Device Regulation) certification. This ...

Synergy with CERT-ASINHPA
Click on the logos to visit the dedicated websites Evolucare and Cert-Asinhpa: a synergy to strengthen digital security in healthcare In a context where cyber attacks targeting healthcare establishments are on the increase, the security of information ...

New ISTQB certifications achieved by our test team
The ISTQB is the International Software Testing Qualifications Board. This organization offers a certification that is recognized worldwide. Achievement of a new ISTQB certification for two of our testers Two members of the team obtained their ...

Medsphère obtains accreditation for INS management
A crucial step for the regulatory requirement coming into force on 1 January 2021 We are pleased to inform you that our Administrative Patient Management (APM) software solution, Medsphère, has obtained INS accreditation following the CNDA review conducted on ...